Answer:
The correct answer is Sample B.
Step-by-step explanation:
The temperature of a system is directly related to the kinetic energy of the molecules. In the case of gases, as the temperature increases, the kinetic energy increases.
The formula of kinetic energy is:
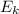 = 1/2 * m *
= 1/2 * m *
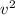
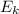 : Kinetic energy of each particle.
: Kinetic energy of each particle.
m: Mass of each particle.
v: Speed of each particle.
For sample A:
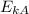 =
=
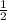 * 28 *
* 28 *
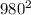
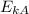 = 13445600
= 13445600
For sample B:
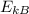 =
=
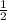 * 44 *
* 44 *
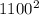
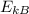 = 26620000
= 26620000
Being
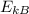 >
>
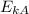 , we can deduce that sample B has a higher temperature.
, we can deduce that sample B has a higher temperature.
Have a nice day!