Conversion factor : molar mass/molecular weight
Mass of H₂O produced = 72 g
Further explanation
Given
Reaction
2H2 + O2- 2H20
8 grams of H2
Required
Conversion factors
Solution
We can use the molar mass of the components and the mole ratio as conversion factors for the above equation
mol H₂ (MW = 2 g/mol) :
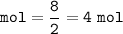
From the equation, mol ratio of H₂ : H₂O = 2 : 2, so mol H₂O = 4 mol
mass H₂O(MW = 18 g/mol) :
mass = mol x MW
mass = 4 x 18
mass = 72 g