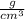Answer:
The density of a sample of gold is 19.32
Step-by-step explanation:
Density can be defined as the property that matter has, whether solids, liquids or gases, to be compressed in a given space. In other words, density is a magnitude that allows you to measure the amount of mass in a given volume of a substance. Then, the expression for the calculation of density is the ratio between the mass of a body and the volume it occupies:
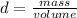
From this expression it can be deduced that the density is inversely proportional to the volume: the smaller the volume occupied by a given mass, the greater the density.
Density, according to the International System of Units, is usually expressed in kilograms per cubic meter (kg/m³) or gram per cubic centimeter (g/cm³).
So, in this case:

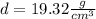
The density of a sample of gold is 19.32