Answer:
1400 millon years, it is probable that if the mineral came from igneous rock, ithe rock age would be the same. There were active volcanoes 1400 year ago in proterozoic era.
Step-by-step explanation:
Lifetime of radiactive compound is calculated with
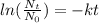 with
with
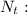 mass at time t,
mass at time t,
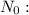 original mass,
original mass,
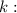 decay constant.
decay constant.
So we know that when 700 millon years pass, the half of original material decays (
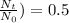 . With that information we can know k.
. With that information we can know k.
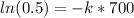 so,
so,
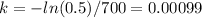
With k, the actual and original quantity of material we can know how old is the rock:
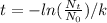 so,
so,
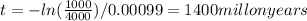
([text]N_0=1000+3000[/text] is the actual quantity plus the quantity that decays to Pb-207)