Answer:
D) He
Step-by-step explanation:
For adiabatic change
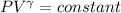
\frac{P_2}{P_1} =(\frac{V_1}{V_2} )^\gamma
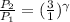
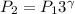
As the value of \gamma increases, P_2 increases.
\gamma for He is 1.67 and \gamma for O₂ IS 1.4
Naturally The value of P_2 is greater for He. Increase in pressure for He is greater.