Answer:
ΔS Rx = -120, 5 J/K
Step-by-step explanation:
The ΔS in a reaction is defined thus:
ΔS Rx = ∑ n S°products - ∑ m S°reactants
For the reaction:
N₂(g) + 2 O₂(g) → 2NO₂(g)
ΔS Rx = 2 mol × 240,5
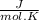 - [ 1 mol × 191,5
- [ 1 mol × 191,5
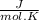 + 2 mol × 205,0
+ 2 mol × 205,0
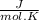 ]=
]=
ΔS Rx = -120, 5 J/K
A negative value in ΔS means a negative entropy of the process. Doing this process entropycally unfavorable.
I hope it helps!