Step-by-step explanation:
Melting point is defined as the point at which a solid substance starts to change into liquid state.
Whereas entropy is the degree of randomness of molecules present in a substance.
Heat of fusion is defined as the amount of heat energy necessary to melt a solid substance at its melting point.
Relation between entropy and heat of fusion is as follows.
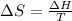
where,
 = 38.98 J/mol K
= 38.98 J/mol K
 = 17.02 kJ/mol
= 17.02 kJ/mol
=
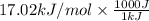
= 17020 J/mol
Therefore, calculate the melting point as follows.
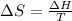
38.98 J/mol K =
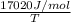
T = 436.63 K
Change the temperature into degree celsius as follows.

=
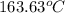
Thus, we can conclude that the melting point in
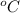 is
is
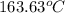 .
.