Step-by-step explanation:
It is known that relation between wavelength and frequency is as follows.
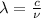
where,
 = wavelength
= wavelength
c = speed of light =
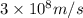
[/tex]\\u[/tex] = frequency
It is given that frequency is
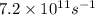 . Hence, putting this value into the above formula and calculate the wavelength as follows.
. Hence, putting this value into the above formula and calculate the wavelength as follows.
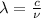
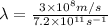
=
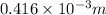
or, =
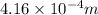
Thus, we can conclude that wavelength of given radiation is
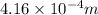 .
.