Answer: The partial pressure of
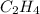 is 281 mmHg
is 281 mmHg
Step-by-step explanation:
We are given:
Solubility of ethene gas =
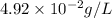
To convert this solubility into mol/L, we divide the given solubility by the molar mass of the gas, which is 28 g/mol
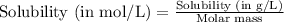
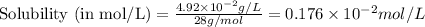
To calculate the molar solubility, we use the equation given by Henry's law, which is:
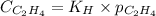
where,
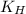 = Henry's constant =
= Henry's constant =
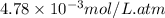
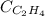 = molar solubility of ethene gas =
= molar solubility of ethene gas =
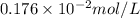
Putting values in above equation, we get:
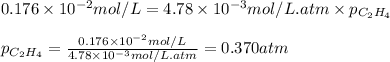
Converting this into mmHg, we use the conversion factor:
1 atm = 760 mmHg
So,
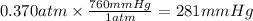
Hence, the partial pressure of
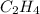 is 281 mmHg
is 281 mmHg