Answer: 11900 J/min (full answer 11948,57 J/min)
Step-by-step explanation:
1) First we want to find the total energy supplied to the 500g of water
Specific heat is the amount of thermal energy you need to supply to a sample weighing 1 kg to increase its temperature by 1 K.
Formula:
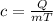
where
c - specific heat capacity (different for each substance) [J/kg K]
Q - Heat energy supplied [J]
m - mass [kg]
T - change in temperature [K]
mass in this question = 500g = 0.5kg
change in temperature = (70 - 30) = 40K (change in Celcius is equal to change in Kelvin)
specific heat capacity of water = 4182 J/kg K (this is just something you can look up in a formula sheet)
now we can rearrange the equation to get Q

Q = 4182 * 0.5 * 40 = 83640 J - this is total heat energy supplied
2) Now we can calculate heat supplied per minute
total energy/number of minutes
time (t) = 7 min
Q/t = 83640/7 = 11948,57 J/min = 11900 J/min (3sf)