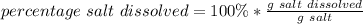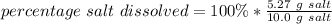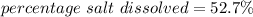Answer:
- The solubility of the salt is 35.16 (g/100 g of water).
- It would take 71.09 grams of water to dissolve 25 grams of salt.
- The percentage of salt that dissolves is 52.7 %
Step-by-step explanation:
a.
We know that 3.20 grams of salt in 9.10 grams of water gives us a saturated solution at 25°C. To find how many grams of salt will gives us a saturated solution in 100 grams of water at the same temperature, we can use the rule of three.
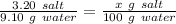
Working it a little this gives us :
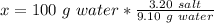
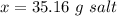
So, the solubility of the salt is 35.16 (g/100 g of water).
b.
Using the rule of three, we got:
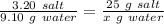
Working it a little this gives us :
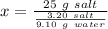
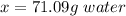
So, it would take 71.09 grams of water to dissolve 25 grams of salt.
C.
Using the rule of three, we got that for 15.0 grams of water the salt dissolved will be:
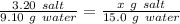
Working it a little this gives us :
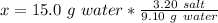
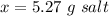
This is the salt dissolved
The percentage of salt dissolved is: