Answer:
For 1: The largest positive cell potential is of cell having 1st and 4th half reactions.
For 2: The standard electrode potential of the cell is 1.539 V
For 3: The smallest positive cell potential is of cell having 3rd and 4th half reactions. The standard electrode potential of the cell is 0.46 V
Step-by-step explanation:
The substance having highest positive
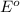 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction.
We are given:
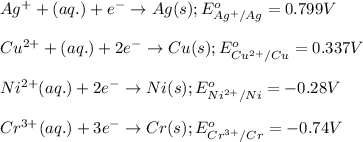
Substance getting oxidized always act as anode and the one getting reduced always act as cathode.
To calculate the
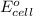 of the reaction, we use the equation:
of the reaction, we use the equation:
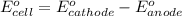
- Cell having 1st and 2nd half reactions:
Silver has higher electrode potential. So, this will undergo reduction reaction and act as anode. Copper will undergo oxidation reaction and act as cathode.
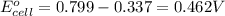
- Cell having 1st and 3rd half reactions:
Silver has higher electrode potential. So, this will undergo reduction reaction and act as anode. Nickel will undergo oxidation reaction and act as cathode.
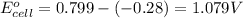
- Cell having 1st and 4th half reactions:
Silver has higher electrode potential. So, this will undergo reduction reaction and act as anode. Chromium will undergo oxidation reaction and act as cathode.
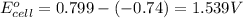
- Cell having 2nd and 3rd half reactions:
Copper has higher electrode potential. So, this will undergo reduction reaction and act as anode. Nickel will undergo oxidation reaction and act as cathode.
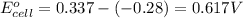
- Cell having 3rd and 4th half reactions:
Nickel has higher electrode potential. So, this will undergo reduction reaction and act as anode. Chromium will undergo oxidation reaction and act as cathode.
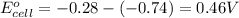
Hence,
For 1: The largest positive cell potential is of cell having 1st and 4th half reactions.
For 2: The standard electrode potential of the cell is 1.539 V
For 3: The smallest positive cell potential is of cell having 3rd and 4th half reactions. The standard electrode potential of the cell is 0.46 V