Answer : The order of reaction with respect to A is, first order reaction.
The order of reaction with respect to B is, zero order reaction.
The overall order of reaction is, first order reaction.
Explanation :
Rate law is defined as the expression which expresses the rate of the reaction in terms of molar concentration of the reactants with each term raised to the power their stoichiometric coefficient of that reactant in the balanced chemical equation.
For the given chemical equation:
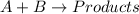
Rate law expression for the reaction:
![\text{Rate}=k[A]^a[B]^b](https://img.qammunity.org/2020/formulas/chemistry/college/5bzomeoytvred365xfytwaqach3ri2hfvj.png)
where,
a = order with respect to A
b = order with respect to B
Expression for rate law for first observation:
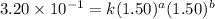 ....(1)
....(1)
Expression for rate law for second observation:
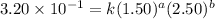 ....(2)
....(2)
Expression for rate law for third observation:
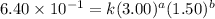 ....(3)
....(3)
Dividing 1 from 2, we get:
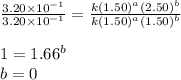
Dividing 1 from 3, we get:
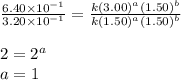
Thus, the rate law becomes:
![\text{Rate}=k[A]^1[B]^0](https://img.qammunity.org/2020/formulas/chemistry/college/kd5pr0f38hvo63qw1p2wq14pxjqho5wgme.png)
![\text{Rate}=k[A]](https://img.qammunity.org/2020/formulas/chemistry/college/70bz13bbmup4nxdfzml5qcbjug1vvhm7gf.png)
Thus,
The order of reaction with respect to A is, first order reaction.
The order of reaction with respect to B is, zero order reaction.
The overall order of reaction is, first order reaction.