Step-by-step explanation:
The given data is as follows.
mass of gasoline = 595.0 lb
volume of gasoline =
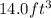
As it is known that density is the amount of mass of a substance divided by its volume.
So, density of gasoline will be as follows.
Density =

=
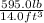
= 42.5
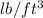
As, 1 lb = 0.4536 kg and 1 ft = 0.3048 m. Now, putting these values into the above equation (1) as follows.
Density = 42.5
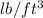
=
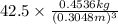
= 680.792
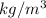
= 680.8
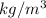 (approx)
(approx)
Density of water is 1000
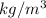 . To measure specific gravity of gasoline, the formula will be as follows.
. To measure specific gravity of gasoline, the formula will be as follows.
specific gravity of gasoline =
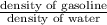
=
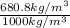
= 0.681
Thus, we can conclude that the specific gravity of gasoline is 0.681.