Answer: 53 kJ/mol
Step-by-step explanation:
Hello, for the chemical reaction you're asking, consider that its balance result:
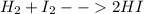
Now, the energy change is given by the difference in the standard enthalpy of formations times the stoichiometric coefficient in the chemical reaction:
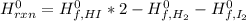
Since hydrogen and iodine are gases, their enthalpy of formation is 0 and the hydrogen iodide has a value of 26.5 kJ/moL, thus, the energy change at standard conditions is given by:
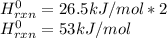
Best regards.