Step-by-step explanation:
The given data is as follows.
Boiler capacity =
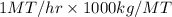 = 1000 kg/hr
= 1000 kg/hr
Steam pressure = 12

For saturated steam, from steam table :
Steam temperature =
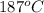
Specific enthalpy of water = 794.65 kJ/kg
Specific enthalpy of steam = 2783.10 kJ/kg
Therefore, difference between enthalpy of steam and water will be calculated as follows.
= 2783.10 - 794.65
= 1988.45 kJ/kg
So, heat required will be calculated as follows.
Heat required =
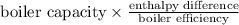
=
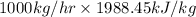
= 1988450 kJ/hr
Also, Fuel required =
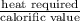
=
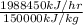
= 13.25 kg/hr
Hence, Volumetric flow rate of hydrogen require =

=
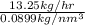
= 147.45
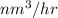
Thus, we can conclude that 147.45
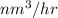 hydrogen as a fuel will be burnt.
hydrogen as a fuel will be burnt.