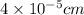Answer :
(a) The energy of blue light (in eV) is 2.77 eV
(b) The wavelength of blue light is
Step-by-step explanation:
The relation between the energy and frequency is:
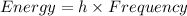
where,
h = Plank's constant =
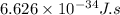
Given :
Frequency =
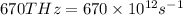
Conversion used :
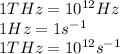
So,
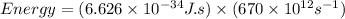
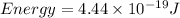
Also,
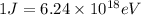
So,
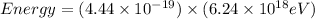
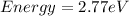
The energy of blue light (in eV) is 2.77 eV
The relation between frequency and wavelength is shown below as:
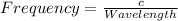
Where,
c = the speed of light =
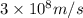
Frequency =
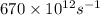
So, Wavelength is:
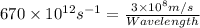
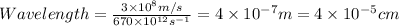
Conversion used :
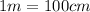
The wavelength of blue light is