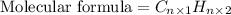Answer:
The corrects answer is last option.
The empirical formula of a compound =
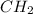
For determining the molecular formula, we need to determine the valency which is multiplied by each element to get the molecular formula.
The equation used to calculate the valency is :
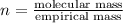
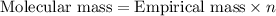
Molecular formula will determined by multiplying the 'n' with subscript numbers of carbon and hydrogen in the empirical formula.