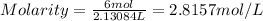Answer:
The molarity of a 6.0 mole% sulfuric acid solution is 2.8157 Molar.
Step-by-step explanation:
Suppose there are 100 moles in solution:
Moles of sulfuric acid = 6% of 100 moles = 6 moles
Mass of 6 moles of sulfuric acid = 6 mol × 98 g/mol=588 g
Moles of water = 100%- 6% = 94%= 94 moles
Mass of water = 94 mol × 18 g/mol = 1692 g
Specific gravity of the solution ,S.G= 1.07
Density of solution = D
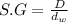
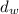 = density of water = 1 g/mL
= density of water = 1 g/mL
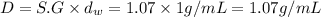
Mass of the solution = 588 g + 1692 g = 2280 g
Volume of the solution = V
Volume =
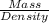
1 mL = 0.001 L
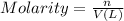
n = number of moles of compound
V = volume of the solution in L
here we have ,n = 6 moles of sulfuric acid
V = 2.13084 L
So, the molarity of the solution is :