Answer:
The last reaction is an oxidation:
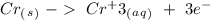
Step-by-step explanation:
For the first half-reaction, we have a strange reduction the charge is not balanced so, it is not a possible reaction. In the second half-reaction we will have a reduction process as such, we have 2 negative charges in both sides and the electrons are placed on the reactive side. For the third half-reaction, we will have strange oxidation because the charge is not balanced, we have a charge of +1 in the left and a charge of -1 in the right, so this is not a possible reaction. For the last half-reaction, we have the production of electrons and the charge is equal on both sides, which is zero.
Finally to remember, Oxidation=production of electrons, Reduction=Consumtion of electrons.