Answer:
4.5 L water we have in litres (L).
Step-by-step explanation:
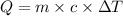
where
 = Final T - Initial T
= Final T - Initial T
Q is the heat energy in calories
c is the specific heat capacity (for water 1.0 cal/(g℃))
m is the mass of water
Plugging in the values

So,
Volume of water = mass/density
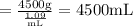
=4.5 L (Answer)