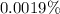Answer:
The percentage of volume taken by iron(II) in the blood cell is 0.0019%.
Step-by-step explanation:
Radius of iron(II) ions ,r= 75.1 pm =
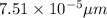
1 pm = 10^{-6} μm
Volume of sphere =
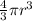
Volume of single iron(II) ion = V
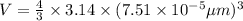
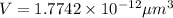
Number of iron(II) ions in one hemoglobin structure = 4
Number of hemoglobin structure in blood cell =
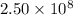 molecules
molecules
Then number of iron (II) ions in
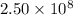 molecules of hemoglobin:
molecules of hemoglobin:
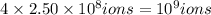
Volume of
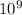 ions of iron =
ions of iron =
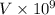
Volume of the hemoglobin structure,V' =
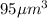
Percentage volume of iron (II) ions in a single blood cell:
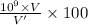
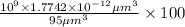
=