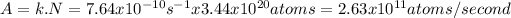Answer:
The activity of 50mg of Sr is
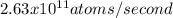
Step-by-step explanation:
Every nuclear disintegration follows the first order rate, thus the half-life (t1/2) is
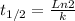 where k is the constant of the reaction.
where k is the constant of the reaction.
Considering 1 year equals 3.15x107 seconds
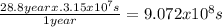
For Sr with t1/2 =
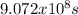 , the constant of the reaction is
, the constant of the reaction is
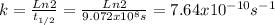
The activity (A) of a nucleus is
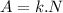 where N is the number of nucleus. Sr molecular mass is 87.6g/mol and every mol contains 6.023x1023 atoms, thus
where N is the number of nucleus. Sr molecular mass is 87.6g/mol and every mol contains 6.023x1023 atoms, thus
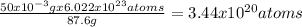
Therefore, the activity of 50mg of Sr is