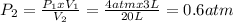Answer:
After the transfer the pressure inside the 20 L vessel is 0.6 atm.
Step-by-step explanation:
Considering O2 as an ideal gas, it is at an initial state (1) with V1 = 3L and P1 = 4 atm. And a final state (2) with V2 = 20L. The temperature remain constant at all the process, thus here applies the Boyle-Mariotte law. This law establishes that at a constant temperature an ideal gas the relationship between pressure and volume remain constant at all time:
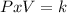
Therefore, for this problem the step by step explanation is:
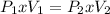
Clearing P2 and replacing