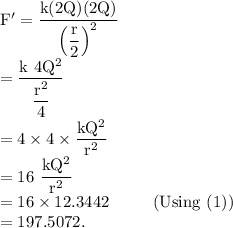Answer:
197.5072.
Step-by-step explanation:
According to the Coulomb's law, the magnitude of the electrostatic force of interaction between two charges
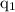 and
and
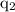 which are separated by the distance
which are separated by the distance
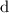 is given by
is given by
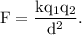
where, k is the Coulomb's constant.
For the case, when,
Then, using Coulomb's law,
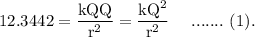
For the case, when,
Then, using Coulomb's law, the new electric force between the charges is given by,