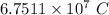Answer:
Step-by-step explanation:
The atomic weight of oxygen = 15.9994 g
This mass corresponds to 1 mole of the oxygen atoms.
Thus,
15.9994 g mass of oxygen contains
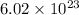 atoms of oxygen.
atoms of oxygen.
1.4 kg = 1400 g ( 1 kg = 1000 g)
So,
1400 g mass of oxygen contains
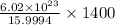 atoms of oxygen.
atoms of oxygen.
Number of atoms in 1400 g of oxygen =
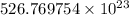
Also, 1 atom of oxygen contains 8 protons
Charge of 1 proton = +
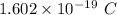
So, Charge on 1 atom of oxygen =
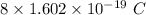
Thus,
Charge on
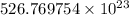 atoms of oxygen =
atoms of oxygen =
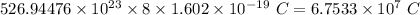
Thus, positive charge in 1.4 kg of oxygen =