Answer: mixing calcium carbonate and HCI
heating copper sulfate pentahydrate
mixing potassium iodide and lead nitrate
combining magnesium and HCI
burning the candle
Step-by-step explanation:
A physical change is defined as a change in which there is alteration in shape, size etc. No new substance gets formed in these reactions. Example: Melting of ice
A chemical change is defined as a change in which a change in chemical composition takes place. A new substance is formed in these reactions. Example: Corrosion of iron
a) crushing calcium carbonate is a physical change as there is only a change in size.
b) mixing calcium carbonate and HCI : is a chemical change as there is formation of new substances.
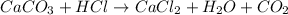
c) boiling water is a physical change as there is only a change in state.
d) heating copper sulfate pentahydrate: is a chemical change as there is breaking of bonds.
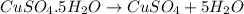
e) separating iron filing and sulfur : is a physical change as there is only separation of components.
f) mixing potassium iodide and lead nitrate: is a chemical change as there is formation of new substances.
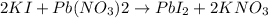
g) combining magnesium and HCI: is a chemical change as there is formation of new substances.
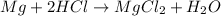
h) burning the candle : is a chemical change as there is formation of new substances.