Answer:
a) 17,20

b) 87,57%
c) 0,7259
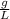
Step-by-step explanation:
a) With molar composition of LNG the average molecular weight is:
3,9%×30,07
 + 1,1%×44,1
+ 1,1%×44,1
 +1,1%×44,01
+1,1%×44,01
 + 93,9%×16,04
+ 93,9%×16,04
 = 17,20
= 17,20

b) The weight fraction of CH₄ is:
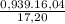 ×100 = 87,57%
×100 = 87,57%
c) It is possible to use ideal gas law thus:
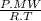 = δ
= δ
Where:
P is pressure: 148 kPa
MW is molecular weight: 17,20

R is gas constant: 8,31
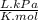
T is temperature: 422K.
And δ is density. Replacing you will obtain:
δ = 0,7258

I hope it helps!