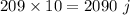Answer:
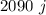 needed to heat the amount of water giving.
needed to heat the amount of water giving.
Step-by-step explanation:
To solve this question we first need to know the specific heat of water which is given by 4.18 joules Per gram for increasing 1° of temperature. Now since one gram of water name one joules per gram so 2 grams will need twice of 4.18 that is 8.36 similarly 50 grams will need
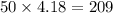
Now we need to calculate the heat for 10° of change because temperature rises from 50° centigrade to 60° centigrade and difference in degrees
so heat needed is=