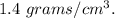Answer: Option: B
The density of the sugar cube is
Step-by-step explanation:
Given,
The given Mass of the sugar cube is =
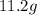
The given each Side of the sugar cube is =
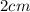
Then the volume of the sugar cube is =
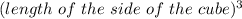
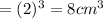
The required Density of the sugar cube is given by =

Then the required density of the sugar cube is=
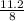
Therefore the required density of the sugar cube is =