Step-by-step explanation:
Manganese(III) oxide is a chemical compound with chemical formula
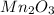 .
.
Since, manganese is a transition metal so, in order to attain stability it will lose valence electrons and as oxygen is chemically combining with it will take up the electrons.
Hence, it will lead to the formation of an ionic compound. Therefore, oxidation state of Mn in this compound is +3 and oxidation state of oxygen is -2.
Therefore, we can conclude that Manganese(III) oxide is a transition metal compound. The oxidation state of manganese in this compound is +3, and the chemical formula of the compound is
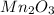 .
.