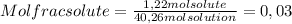Answer:
a) 1,74 molal
b) 37,2 %
c) 0,03
Step-by-step explanation:
We are going to define sucrose as solute, water as solvent and the mix of both, the solution.
Let´s start with the data:
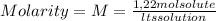
We can assume as a calculus base, 1 liter of solution. So, in 1 liter of solution we have 1,22 moles of solute:
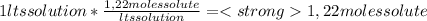
Knowing that the molality (m) is defined as mol of solute/kgs solvent, we have to calculate the mass of solvent on the solution. Remember our calculus base (1 lts of solution). In 1 lts of solution we have 1120 grams of solution.
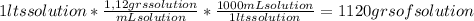
With the molecular weight of solute (Sum of: for carbon = 12*12=144; for hydrogen = 1*22=22 and for oxygen = 16*11=176. Final result = 342 grs per mol), we can obtain the mass of solute:
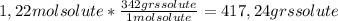
Now, the mass of solvent is: mass solvent = mass of solution - mass of solute. So, we have: 1120 - 417,24 = 702,76 grs of solvent = 0,70276 Kgs of solvent
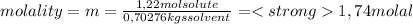
For b) question we have that the mass percent of solute is hte ratio between the mass of solute and the mass of solution. So,

For c) question we have that the mole fraction of solute is the ratio between moles of solute and moles of solution. Let's calculate the moles of solution as follows: Moles solution = moles solute + moles solvent. First we have that the moles of solvent are (remember that the molecular weight of water for this calculus is 18 grs per mol):
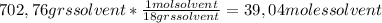
So, we have the moles of solution: 1,22 moles of solute + 39,04 moles of solvent = 40,26 moles of solution
Finally, we have: