Answer:
220.44g Ba²⁺ ions in solution
Step-by-step explanation:
Given parameters:
Mass of barium nitrate = 61.2g
Volume of solution = 1 liter
Unkown:
Mass of barium in 7.5quarts of solution?
Solution
We must first convert quarts to its liter equivalence:
1 quarts = 0.95 liter
7.5 quarts = 0.95 x 7.5; 7.125liter
Now, let us find the mass of barium nitrate in a solution of 7.125liter:
Given:
1 liter of solution contains 61.2g of barium nitrate:
7.125 liter will contain 7.125 x 61.2 = 436.05g of barium nitrate.
The formula of the compound is Ba(NO₃)₂:
In solution we have Ba²⁺ + NO₃⁻
Ba(NO₃)₂ → Ba²⁺ + 2NO₃⁻
Number of moles of Ba(NO₃)₂ =
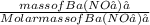
Molar mass of Ba(NO₃)₂ = 137 + 2[14 + 3(16)] = 271g/mol
Number of moles of Ba(NO₃)₂ =
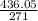 = 1.609mole
= 1.609mole
1 mole of Ba(NO₃)₂ will produce 1 mole of Ba²⁺ ions in solution
therefore, 1.609mole of Ba(NO₃)₂ will also yield 1.609mole of Ba²⁺ ions in solution
Mass of Ba²⁺ ions = Number of moles of Ba²⁺ ions x molar mass of Ba²⁺ ions
Mass of Ba²⁺ ions = 1.609 x 137 = 220.44g