Answer:
a) Reversible processes
b) 1222.73 J/K and 392.144 J/K
c) Because of breaking bonds that are a stable state, while accelerating molecules in the other is less difficult.
Step-by-step explanation:
Since it is equality, it should be applied to reversible processes, and even better to isothermal processes, since the Temperature remains constant. The T is the absolute temperature (measured in °K) and dQ is the heat absorbed by the system, which DEPENDS on the process.
b) The heat absorbed in the fusion process depends on the latent heat of fusion, L, of the water, which is 334 J/g. It says t kg, but I assume it was a mistake in the typing, so the change in entropy is calculated for 1 kg of water melting as follows:
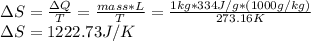
Now, to proceed with the change of entropy for water heated from 273.16K to 300K we use the specific heat of water, which is 4184 J/kg°K as follows:
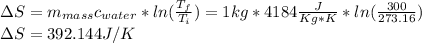
c) In the solid state, water molecules have different bonds with other water molecules creating the crystals. In the liquid state, each molecule moves freely with less interaction between molecules. So, it required more energy to break these bonds and alter this ordered state than just accelerating the molecules in the liquid state.