Answer:
change in internal energy 3.62*10^5 J kg^{-1}
change in enthalapy 5.07*10^5 J kg^{-1}
change in entropy 382.79 J kg^{-1} K^{-1}
Step-by-step explanation:
adiabatic constant
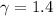
specific heat is given as

gas constant =287 J⋅kg−1⋅K−1

specific heat at constant volume

change in internal energy


change in enthalapy


change in entropy


