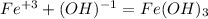Answer:
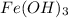
Step-by-step explanation:
The problem gives you the systematic name for the Iron (III) hydroxide, it means that the Iron in the compound is using its oxydation number +3, that is:
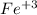
And as an hydroxide, has the hydroxide ion, which oxidation number is -1:
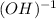
To form the Iron (III) hydroxide there are the iron and the hydroxide ion, and their oxidation numbers take its position below the opposite ion, that is: