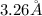Answer: The de-Broglie's wavelength of a hydrogen molecule is
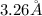
Step-by-step explanation:
Kinetic energy is the measure of temperature of the system.
The equation used to calculate kinetic energy of a particle follows:
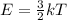
where,
E = kinetic energy of the particles = ?
k = Boltzmann constant =
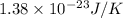
T = temperature of the particle = 30 K
Putting values in above equation, we get:
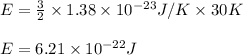
- Calculating the mass of 1 molecule of hydrogen gas:
Conversion factor used: 1 kg = 1000 g
1 mole of hydrogen gas has a mass of 2 grams or
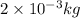
According to mole concept:
 number of molecules occupy 1 mole of a gas.
number of molecules occupy 1 mole of a gas.
As,
 number of hydrogen molecules has a mass of
number of hydrogen molecules has a mass of
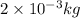
So, 1 molecule of hydrogen will have a mass of =
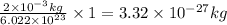
- To calculate the wavelength of a particle, we use the equation given by De-Broglie's wavelength, which is:
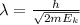
where,
 = De-Broglie's wavelength = ?
= De-Broglie's wavelength = ?
h = Planck's constant =
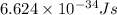
m = mass of 1 hydrogen molecule =
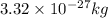
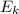 = kinetic energy of the particle =
= kinetic energy of the particle =
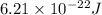
Putting values in above equation, we get:
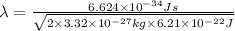
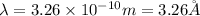 (Conversion factor:
(Conversion factor:
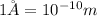 )
)
Hence, the de-Broglie's wavelength of a hydrogen molecule is