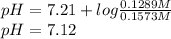Answer:
The pH of the drink is 7.12
Step-by-step explanation:
First, we calculate the concentration of NaH₂PO₄ and Na₂HPO₄, using their molecular weight and the volume in L (355 mL= 0.355 L):
[NaH₂PO₄] =
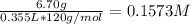
[Na₂HPO₄] =
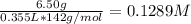
Now we calculate the pH of the solution, keeping in mind the equilibrium:
From literature, we know that the pka for the previous equilibrium is 7.21
The equation that gives us the pH of a buffer solution is the Henderson–Hasselbalch equation:
pH = pka +
![log([HPO4^(-2) ])/([H2PO4^(-) ])](https://img.qammunity.org/2020/formulas/chemistry/college/ubv1d58d9do55fsla4mmd4xcc3f9m35g3z.png)
Replacing in the equation the data we know gives us: