Answer:48.52 kJ
Step-by-step explanation:
Given
Resistance
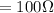
temperature increases from
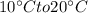
Voltage=50 V
Heat given(H)
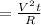
Where V=voltage applied
t=time
R=Resistance
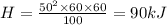
Heat absorbed by water is
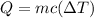
where
m=mass of water
c=specific heat of water
 =change in temperature
=change in temperature
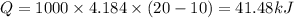
Therefore 90-41.48=48.52 kJ is not absorbed by water and leaves the system into the surroundings.