Answer:
N₂ is the limiting reactant
Step-by-step explanation:
The balanced reaction between N₂ gas and H₂ gas is:
N₂ + 3H₂ → 2NH₃
In order to determine the limiting reactant, we have to calculate the number of moles of each rectant, using their molecular weight:
- Moles of N₂= 100 kg *
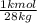 = 3.57 kmol
= 3.57 kmol - Moles of H₂= 100 kg *
 = 50.0 kmol
= 50.0 kmol
Lastly, we multiply the number of moles of N₂ by 3, and the number of moles of H₂ by 1; due to the coefficients in the balanced reaction. Whichever number is lower, belongs to the limiting reactant.
N₂ => 10.7
H₂ => 50.0
Thus N₂ is the limiting reactant