Answer:
45.8 kg
Step-by-step explanation:
Given that the mole fraction of water = 0.752
For a binary system,
The mole fraction of water + The mole fraction of ethanol = 1
So,
The mole fraction of ethanol = 0.248
Given that the total mass = 100 kg
Let the mass of ethanol = x kg
The mass of water = 100 - x kg
The formula for the calculation of moles is shown below:

Molar mass of ethanol = 46.07 g/mol
Molar mass of water = 18 g/mol
Also, 1 g = 10⁻³ kg
So,
Molar mass of ethanol = 46 ×10⁻³ kg/mol
Molar mass of water = 18 ×10⁻³ kg/mol
Moles of ethanol = x / 46 ×10⁻³ moles
Moles of water = (100 - x) / 18 ×10⁻³ moles
So, according to definition of mole fraction:
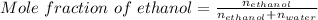
Applying values as:
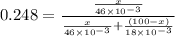
Solving for x, we get
x = 45.8 kg
Mass of ethanol in mixture = 45.8 kg