Answer:
47 mL
Step-by-step explanation:
The equilibrium of nitrous acid is:
HNO₂ ⇄ NO₂⁻ + H⁺ Ka = 4,5x10⁻⁴
If desire pH is 2,60 the [H⁺] concentration in equilibrium is:
[H⁺] =
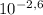 =2,51x10⁻³ M
=2,51x10⁻³ M
Initial molarity of sodium nitrite is:
43,8g ×
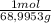 ÷ 2,5 = 0,254 M
÷ 2,5 = 0,254 M
Thus, in equilibrium the concentration of chemicals is:
[NO₂⁻] = 0,254 - x
[HNO₂] = x
[H⁺] = 2,51x10⁻³ = Y-x Where Y is initial concentration.
Equilibrium formula is:
4,5x10⁻⁴ =
![([2,51x10^(-3)[0,254 - x] ])/([x])](https://img.qammunity.org/2020/formulas/chemistry/college/cnxqbvjtatp91lqek3jgfvoc2908gu11xy.png)
Solving, x = 0,215
Thus, initial [H⁺] concentration is:
0,215 + 2,51x10⁻³ = 0,2175 M
If total volume is 2,50 L:
2,50L ×
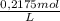 = 0,5438 mol of HCl
= 0,5438 mol of HCl
As molarity of concentrated hydrochloric acid is 11,65 mol per liter:
0,5438 mol HCl ×
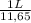 = 0,047 L ≡ 47 mL
= 0,047 L ≡ 47 mL
I hope it helps!