Answer:
The chemical energy of the battery was reduced in 10800J
Step-by-step explanation:
The first thing to take into account is that the stored energy in a battery is in Watts per second or Joules (
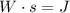 ). It means that the battery provides a power for a certain time.
). It means that the battery provides a power for a certain time.
The idea is to know how much
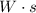 has been consumed by the circuit.
has been consumed by the circuit.
The first step is to know the power that is consumed by the circuit. It is
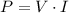 . The problem says that the circuit consumes a current of 5.0A with a voltage of 6.0V. It means that the power consumed is:
. The problem says that the circuit consumes a current of 5.0A with a voltage of 6.0V. It means that the power consumed is:
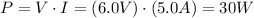
The previous value (30W) is the power that the circuit consumes.
Now, you must find the total amount of power that is consumed by the circuit in 6.0 minutes. You just have to multiply the power that the circuit consumed by the time it worked, it means, 6.0 minutes.
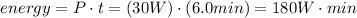
You must convert the minutes unit to seconds. Remember that 1 minute has 60 seconds.
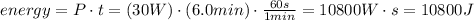
Thus, the chemical energy of the battery was reduced in 10800J