Answer:
Pressure in duct = 799.75 mmHg
Atmospheric pressure = 774.75 mmHg
Gauge pressure = 24.99 mmHg
Step-by-step explanation:
First of all, it is needed to set a pressure balance (taking in account that diameter of manometer is constant) in the interface between the air of the duct and the fluid mercury.
From the balance in the sealed-end manometer, we have the pressure of air duct as:
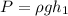
We have that ρ is density of mercury and g is the gravity
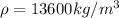

So, replace in the equation:
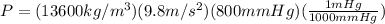
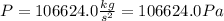
Transforming from Pa to mmHg
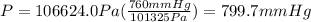
From the balance in the open-end manometer, we have the pressure of air duct as:
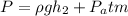
Isolate
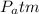 :
:
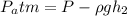
Calculating:
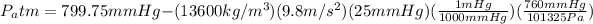

Finally, gauge pressure is the difference between duct pressure and atmospheric pressure, so:
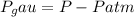
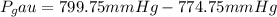
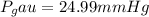
End.