Answer:
- 690 kg of water.
- 125 kg of KCl crystals are formed after cooling process
Step-by-step explanation:
1) A saturated solution is a chemical solution containing the maximum concentration of a solute dissolved in the solvent. The solubility of KCl at 370K is 42%mass -42kg of KCl in 100kg of water+KCl , thus, the amount of water added to 500kg of KCl is:
500 kg of KCl ×
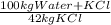 = 1190 kg of water +KCl
= 1190 kg of water +KCl
1190 kg of water +KCl - 500 kg of KCl = 690 kg of water
2) The maximum amount that this solution could solubilize of KCl at 320K is:
1190 kg of water + KCl ×
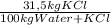 = 375 kg
= 375 kg
Thus, the mass of KCl crystals formed are:
500 kg of KCl - 375 Kg of KCl = 125 kg of KCl
I hope it helps!