Step-by-step explanation:
The given data is as follows.
Mass of a lead atom =
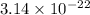
Volume = 2.00

Density = 11.3
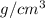
As it is mentioned that 1 cubic centimeter contains 11.3 grams of lead.
So, in 2 cubic centimeter there will be
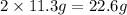 of lead atoms.
of lead atoms.
One lead atom has a mass of
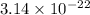 . Therefore, number of atoms present in 22.6 g of lead will be as follows.
. Therefore, number of atoms present in 22.6 g of lead will be as follows.
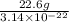
=
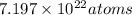
Thus, we can conclude that there are
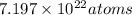 of lead are present.
of lead are present.