Answer:
The amount of anhydrous crystal are coming out of the solution when this is cooled from 80°C to 30°C are 5 kg of A
Step-by-step explanation:
A saturated solution is a chemical solution containing the maximum concentration of a solute dissolved in the solvent, and knowing the solubility of component A at 80°C it is possible to know their amount, thus:
10Kg of water ×
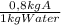 = 8 kg of A
= 8 kg of A
The maximum concentration that water can dissolve at 30°C is:
10Kg of water ×
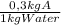 = 3 kg of A
= 3 kg of A
Thus, the amount of anhydrous crystal are coming out of the solution when this is cooled from 80°C to 30°C are:
8 kg of A - 3 kg of A = 5 kg of A
I hope it helps!