Answer: The volume of 0.10 M NaOH required to neutralize 30 ml of 0.10 M HCl is, 30 ml.
Explanation:
According to the dilution law,

where,
 = molarity of stock
= molarity of stock
 solution = 12.0 M
solution = 12.0 M
 = volume of stock
= volume of stock
 solution = 5.0 ml
solution = 5.0 ml
 = molarity of dilute
= molarity of dilute
 solution = 0.2 M
solution = 0.2 M
 = volume of dilute
= volume of dilute
 solution = ?
solution = ?
Putting in the values we get:
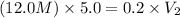
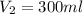
Therefore, 300 ml of 0.2M HCl can be made from 5.0mL of a 12.0M
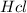 solution.
solution.