Answer: A. The increase in the mass of the magnesium oxide was due to oxygen atoms in the air.
Step-by-step explanation:
According to the law of conservation of mass, mass can neither be created nor be destroyed. Thus the mass of products has to be equal to the mass of reactants. The number of atoms of each element has to be same on reactant and product side. Thus chemical equations are balanced.
The balanced chemical equation for burning of magnesium in air is:
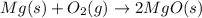
When magnesium combines with oxygen, the mass of magnesium oxide formed will be same as the sum of masses of magnesium and oxygen.
Thus the increase in the mass of the magnesium oxide was due to oxygen atoms in the air.