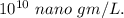Answer:
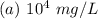
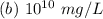
Step-by-step explanation:
(a) As given in question,
concentration of zinc in atmosphere = 10 g/L
By converting g to mg,
1 g = 1000 mg
=> 10 g = 10 x 1000 mg
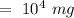
Hence, the concentration of zinc in the environment in mg/L is
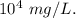
(a) As given in question,
concentration of zinc in atmosphere = 10 g/L
By converting g to nano gm,
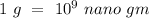
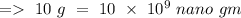
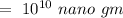
Hence, the concentration of zinc in the environment in nano gm/L is